
It's the most beautiful time of the year and this Smart Friday is a Happy Holidays Season Edition! Let's talk about ... The Geometry of Snowflakes!

As snowflakes gently meander past your living room window this winter, take a break from your hot cocoa and consider nature’s ice-cold architecture. When water molecules chill down, they assemble into a myriad of spectacular shapes from simple hexagons to star-shaped dendrites. Inspect these frozen fractals and you’ll find a recurring theme: the number six. Six sides, six edges, six branches — ice crystals seem six obsessed.
In 1611, German mathematician Johannes Kepler speculated in a New Year’s gift to a friend that this numerical repetition stemmed from the microscopic scale. Water molecules freeze as hexagons, he proposed, which then stack in alternating rows that form more hexagons as additional water molecules join the crystal. Scientists now know that H2O takes on a six-sided structure because of the way hydrogen bonds link water molecules. Kepler’s crystal ponderings laid the groundwork for the field of crystallography, which famously helped reveal the architecture of DNA and now investigates the structure of everything from diamonds to viruses.
Ice arises in an ordered manner, but conditions dictate what shape a crystal takes, and those shapes can vary dramatically. In 1966, Japanese meteorologists Choji Magono and Chung Woo Lee established 80 unique classifications of ice crystals, including cups, needles, bullets and scrolls.

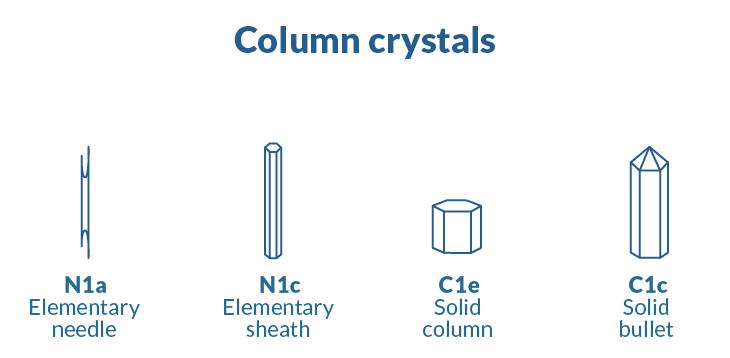
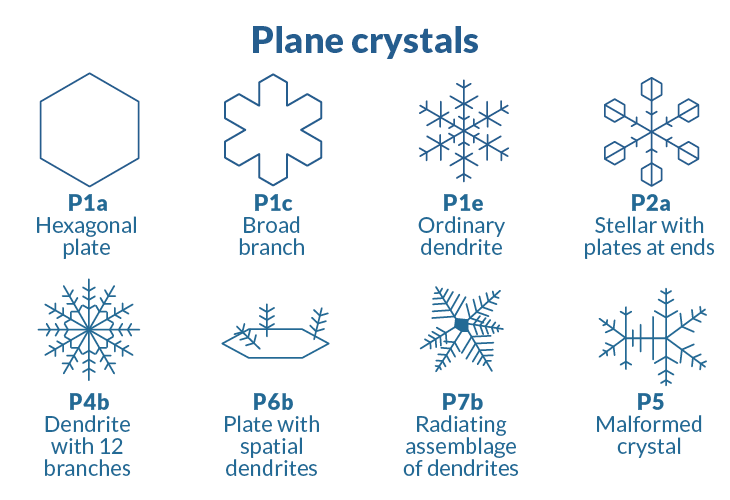 When water molecules chill down, they assemble into a myriad of spectacular shapes, from simple hexagons to star-shaped dendrites.
When water molecules chill down, they assemble into a myriad of spectacular shapes, from simple hexagons to star-shaped dendrites.
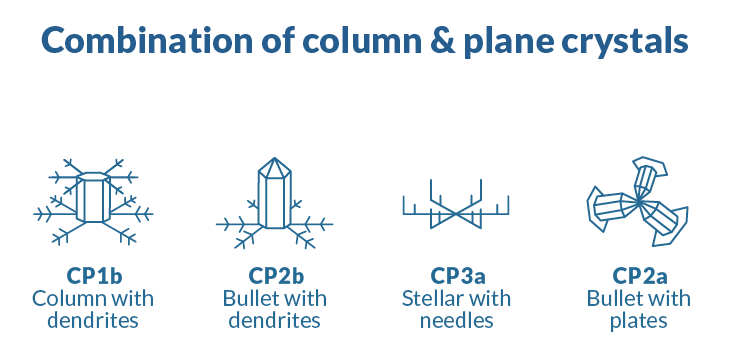

Next time you catch a snowflake on your tongue, think about how geometry, meteorology and the quirky properties of water molecules have conspired to create one of nature’s most spectacular set of minisculptures.
Check the original article here:https://www.sciencenews.org/sponsored/classifying-geometry-snowflakes-highlights-their-beauty-and-diversity
Want to learn more?
Keep reading our Smart Fridays articles! and check all the different lessons about geometry on our learning platform Engage!K12

Check more Smart Fridays articles!
- Smart Friday with RobotLAB -Design Thinking 5 Stage Process
- Smart Friday with RobotLAB - Light: Wave-particle duality
- Smart Friday with RobotLAB - History of Virtual Reality
- Smart Friday with RobotLAB - Optical illusions
- Smart Friday with RobotLAB -Computer Science Vs Computer Programming Differences
- Smart Friday with RobotLAB - Learning how to create your own story!
- Smart Friday with RobotLAB - Let's talk about Gravity!
- Smart Friday with RobotLAB! - Triangle Inequality Theorem
- Smart Friday with RobotLAB- Autonomous Cars



